Geref Sermorelin Acetate Therapy
Geref is one of the many forms of Sermorelin Acetate injection that have been released in the American market.
Sermorelin at a Glance
 Sermorelin is a synthetic form of Growth Hormone-Releasing Hormone. GH-RH comprises a chain of forty-four amino acids, whereas Sermorelin only contains twenty-nine amino acids.
Sermorelin is a synthetic form of Growth Hormone-Releasing Hormone. GH-RH comprises a chain of forty-four amino acids, whereas Sermorelin only contains twenty-nine amino acids.
Despite this fact, Sermorelin functions identically to GH-RH in the human body and is one of the most popular forms of GH-RH therapy.
One of the most common ways Sermorelin is configured for human injection is by forming an acetate salt to which Sermorelin is attached.
Sermorelin Acetate Breakdown
Noted in molecular terms, Sermorelin Acetate is C149 H246 N44 O42 S1. Sermorelin acetate has a weight of 3,358 daltons.
Geref is sterilized and has been proven not to produce an inflammatory response when injected into the human body.
Geref is freeze-dried through sublimation in reconstituted form, allowing it to be transported more effectively and stored for a more extended period than its liquid form.
Before Geref is injected, it must be reconstituted, combining the lyophilized Sermorelin Acetate with a Sodium Chloride Solution. After reconstitution, the mixture has a pH of between 5 and 5.5.
How is Geref Distributed?
Geref is delivered to the patient via vials. The contents of the vial are as follows:
Each half-milligram vial holds 0.5 milligrams of sermorelin acetate and 5 milligrams of mannitol. The pH is fixated using a monobasic sodium phosphate buffer and dibasic sodium phosphate.
Each one-milligram vial holds 1.0 milligrams of sermorelin acetate and 5 milligrams of mannitol. These contents have pH levels that are also fixated using monobasic sodium phosphate and dibasic sodium phosphate.
How does Geref Work?
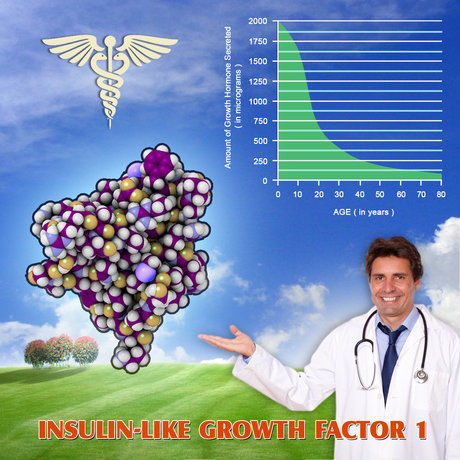
Geref is an injectable form of Sermorelin Acetate, which has the ability to boost plasma levels of Insulin-Like Growth Factor One and Human Growth Hormone by stimulating the endogenous production of HGH by the pituitary gland.
Geref functions in the body identically to endogenous GH-RH, stimulating HGH production in an identical manner in humans.
How does Geref interact with the Human Body?
Distribution
After 0.25 to 1-milligram intravenous injection of Human Growth Hormone was delivered to twelve regular patients, the mean volume distribution ranged from 23.7 to 25.8 liters.
Absorption
After two milligrams of Sermorelin were subcutaneously injected into twelve regular patients, peak Sermorelin saturation occurred between five and twenty minutes after injection. After this administration, the absolute mean bioavailability of sermorelin was  around six percent.
around six percent.
Metabolism
There has been no human research regarding Geref.
Evacuation
Sermorelin leaves the body quite quickly after it has entered the bloodstream. In adults, Sermorelin is cleared from the body at a rate of around 2.6 liters per minute. Geref also has a short half-life of around eleven to twelve minutes after its initial subcutaneous or intravenous injection.
Among Individual Populations
Age/Gender: In healthy adults, Geref is evacuated from the body at a similar rate in both men and women. There is no clearance data related to the patient's age. There is also data that compares male and female pediatric clearance rates.
Hepatic/Renal Insufficiency: There is no available research on how liver and kidney problems may affect clearance rates.
Clinical Evaluation of Geref
In one particular non-blind clinical research study performed across multiple medical centers, prepubescent adolescents suffering from idiopathic HGH Deficiency were given Geref injections of 30 micrograms per kilogram daily via SC injection.
After one year of treatment, 56 participants were available to evaluate efficacy after twelve months. Another 54 participants were not qualified for evaluation.
Twenty-four violated criteria eligibility, ten discontinued the required protocol for treatment, and twenty others did not meet the criteria for evaluation after the initial six months of the study.
Fifty-six of the total 110 participants, including 47 of the 56 patients who were among the subset of participants who were qualified for evaluation and continued Sermorelin Acetate therapy over the course of an entire year, were shown to grow in height at a rate of two centimeters per year faster than their baseline rate of growth.
Among those 56 participants who qualified for evaluation, their height velocity changed at a rate of 4.1 centimeters per year after six months. It increased at a rate of 7.2 centimeters per year after one year.
The average patient grew taller at a rate of 3.1 centimeters per year. The bone age of these patients after one year of Geref therapy was proportional to their height gains.
When should Geref be used?
Geref injections are intended for pediatric patients who suffer from HGH Deficiency with no discernible cause, which results in a failure to grow properly. Most of these pediatric patients who suffer from HGH Deficiency still have active pituitary glands that  respond readily to GH-RH.
respond readily to GH-RH.
Pediatric patients who use Geref should be prepubescent. Geref Therapy should be instituted at a bone age of eight in males and seven and a half in females.
Geref Contraindications
Geref should not be utilized by individuals who have been proven to have an allergy or sensitivity to Sermorelin. It should also be avoided by patients with a sensitivity or allergy to any of the other ingredients included in the product.
Geref Warning
It is vitally important to use Geref immediately after reconstituting with the accompanying diluent. The reconstituted solution will begin to degrade a short time after reconstitution.
If any solution is left over after the injection is complete, it should be thrown away, and under no circumstances should you consider using it later.
Geref Precautions
Geref should only be used with the express consent of a physician specializing in either Growth Disorders or Anti-Aging Therapy. When prescribed, hormone levels should be carefully monitored to ensure that proper hormone levels are restored.
Young patients undergoing Geref Hormone Replacement Therapy must also be evaluated to ensure that Geref produces height gains.
Some patients will not respond to Geref or other GH-RH analogs even if they are not growing correctly. Those pediatric patients who do not respond to Geref therapy will more than likely be fully served using Human Growth Hormone HRT.
It has been thoroughly proven that Geref produces positive changes in height following a single year of treatment; however, there has been no research as to the extent of the effect of Geref upon a patient's final height as an adult.
Geref and Hypothyroidism
In clinical research, 6.5% of participants were diagnosed with hypothyroidism due to Geref therapy. In the most significant Geref clinical trial, 8 participants among 110 who were enrolled were taking thyroid HRT before being administered Geref. 5 more participants began Thyroid Replacement HRT after treatment began.
If a patient suffers from undetected hypothyroidism, it can negate or diminish the effects of Geref Hormone Replacement Therapy. For this reason, the administering physician should test for proper thyroid function before and periodically during Geref treatment.
If at any point before or during therapy, the patient begins to show signs of hypothyroidism, Thyroid Hormone HRT should be instituted as soon as possible to maximize the effectiveness of therapy and the patient's health.
In addition to Thyroid Hormone testing, patients who are undergoing Geref Hormone Replacement Therapy should also be tested periodically for bone age. This is particularly important among patients currently undergoing puberty or concurrently receiving Thyroid Hormone HRT.
In these particular patients, the epiphyseal plates can sometimes grow at a very fast rate. It is essential to suspend Geref Sermorelin Acetate Therapy once the epiphyseal plates close because, at this point, the bones will no longer grow in length, and Geref Therapy will no longer be needed.
Geref Hormone Replacement Therapy is not recommended for patients who suffer from an HGH Deficiency which resulted from an intracranial lesion. There has been no testing as to the safety of Sermorelin Acetate Therapy in this subset of patients.
Reactions to Geref
As is the case with administering any Hormone Replacement Therapy, systemic or local allergic responses can sometimes result. It is vital that the patient (and parent, if applicable) be aware that such responses can potentially occur.
If the patient suffers an allergic reaction as a result of Geref Therapy, it is vital that they receive medical attention promptly.
Clinical Tests have shown that increased blood-serum levels of Insulin-Like Growth Factor One (IGF-1), Human Growth Hormone, alkaline phosphatase, and inorganic mineral phosphorus can occur as a result of Geref Sermorelin Acetate Therapy.
Drug Interactions
Taking glucocorticoid steroids in combination with Sermorelin Acetate Therapy maybe reduce the effectiveness of Geref.
In clinical studies of Geref Sermorelin Acetate Therapy, no pediatric patient who concomitantly took medications to treat normal youthful illnesses/problems had any problems due to combining their standard medications with Geref Hormone Replacement Therapy.
Though no evidence has been discovered, it is still important to note that there have been no clinical studies regarding formal medical interactions.
Fertility Impairment, Mutagenesis, Cancer risk
There has been no longitudinal animal research regarding fertility impairment or carcinogenicity risk regarding Geref Hormone Replacement Therapy. There has been absolutely no clinical research linking Geref Sermorelin Acetate Therapy to genetic abnormalities.
Pregnancy
There has been some animal research conducted regarding Geref Therapy. At a dosage somewhere between three and six times the usual daily dosage that a human patient receives adjusted for physical surface area, minor fetal changes occurred in rabbits and rats.
There have been no adequately controlled studies regarding the usage of Geref by pregnant women. Geref Sermorelin Acetate Therapy should only be administered to pregnant women if the therapy's likely benefits outweigh the possible risk to the unborn child.
Nursing Women
It is unknown if Geref is produced in human milk. The mother releases many medications in nursing, so mothers and physicians should exercise caution when using Geref Hormone Replacement Therapy while nursing.
Patient Information
All patients prescribed Geref (along with their parents, if applicable) should be adequately informed of the possible risks and benefits related to the therapy. If the prescribing physician has determined that Geref should be administered by either the patient or parent at home, the physician must provide appropriate guidance regarding its use.
This includes verbally discussing the content of the Patient Information Booklet with the patient (and parent, if applicable). The Insert accompanying the prescription is meant to assist the patient in the efficient and safe usage of Geref.
patient (and parent, if applicable). The Insert accompanying the prescription is meant to assist the patient in the efficient and safe usage of Geref.
It does not provide complete and total disclosure of all potential, intended, or adverse effects.
Disposal Information
If the physician approves home usage, the patient should be provided with or directed to a location that provides SHARPS containers for proper disposal of used needles and syringes accumulated due to Geref Therapy.
These containers are puncture-resistant and a necessary safety measure to protect patients and anyone who may come in contact with the used needles and syringes.
It is vital that the patient (and parent, if applicable) be directed thoroughly as to the vital importance of proper needle disposal. Also, they should be informed of the dangers of reusing syringes and needles as well.
Side Effects
Many Geref Sermorelin Acetate HRT patients have antibodies against Growth Hormone Factor during at least one therapy point. There is no clear assessment of the significance of the presence of these antibodies, and the levels of these antibodies can change quickly from test to test.
A positive result at one juncture regularly turns negative after the next test. The production of these antibodies does not seem to have any adverse effect on the patient.
Also, these antibodies do not seem to produce any change in the effectiveness of Geref therapy. There have been no reported general allergic responses to Geref Sermorelin Acetate Therapy.
The most common reaction to Geref Hormone Replacement Therapy which is related to treatment, is local irritation around the injection site, which occurs in around one of every six patients. This irritation is characterized by redness, pain, or swelling.
Though this side effect is relatively common, only a tiny minority of patients find the irritation bothersome enough to suspend therapy.
Out of a sample of 350 patients who underwent Geref Therapy in a clinical trial, only three suspended therapy due to injection-site irritation. Other side effects occurred in less than one percent of patients.
These side effects include severe drowsiness, hives, vomiting, headache, nausea, difficulty swallowing, hyperactivity, chest tightness, pallor, distortion in taste perception, and skin flushing.
Geref Dependency and Abuse:
There is no evidence to suggest that using Geref for any time will result in any dependency or proclivity toward abuse. The general pharmacology of Geref does not produce any addictive effect, and clinical trials have produced no evidence of such an effect.
Never Take More than Prescribed
It is not recommended to exceed your physician's recommended dosage of Geref. Overdose will not provide better results. It will only increase the occurrence of side effects.
Administration and Dosage
The recommended dosage for pediatric patients for Geref Sermorelin Acetate Therapy is 0.03 milligrams per kilogram of body weight. This dosage should be administered daily before bed (unless otherwise advised by your physician) via subcutaneous injection. Also, injection sites should be rotated periodically to reduce the risk of irritation.
In pediatric patients who are prescribed Geref Sermorelin Acetate Therapy, therapy should be discontinued after the epiphyseal plates have fused. Patients who do not respond to Geref Treatment should undergo further evaluation to discover the root cause of their unresponsiveness to therapy.
Patients who do not experience increased levels of Human Growth Hormone as a result of therapy likely suffer from some other ailment or deficiency which prevents them from producing sufficient HGH levels.
Regular Height Assessment is Important
Pediatric patients should undergo a height assessment at least twice yearly as they continue Geref Therapy. It is crucial that children who take Geref experience growth rates that are consistent with both their stage of development and their age.
If Geref Sermorelin Acetate Treatments is discovered to be inadequate, other forms of therapy should be considered. HGH Hormone Replacement Therapy is the most common consideration for patients who have a waning or poor response to Ger
ef Treatments.
Avoid Contamination of Geref Sermorelin Acetate Therapy
Clean the rubber stopper containing the reconstituted solution before piercing it with the needle to avert contamination risk. It is also strongly recommended that Geref be injected using disposable and sterile needles and syringes.
The syringe should be small enough to where the proper dose of Geref can be drawn with reasonably high accuracy.
After the correct dose has been determined, the vial of Geref should be reconstituted using 0.5-1.0 milliliters of NaCl solution.
To reconstitute Geref Sermorelin Acetate, insert the diluent into the Geref vial via injection. Tilt the vial in a manner so that the liquid rolls down the wall of the vial onto the active ingredient below. Gently swirl the container in a circular motion until the solution completely dissolves.
IMPORTANT: Geref should not be administered if visible particles are in the solution after reconstitution, nor should it be used if the resultant reconstitution is cloudy.
 Before Geref Therapy is Reconstituted for use, it must be refrigerated at a temperature between 36 and 46 degrees Fahrenheit (2 and 8 degrees Celsius).
Before Geref Therapy is Reconstituted for use, it must be refrigerated at a temperature between 36 and 46 degrees Fahrenheit (2 and 8 degrees Celsius).
Expiration dates are also clearly shown on the label. If the Vial becomes unrefrigerated or becomes expired, the treatment should be discarded.
Contact Us For A Fast And Professional Response

- Sermorelin Acetate Side Effects [Last Updated On: February 12th, 2025] [Originally Added On: September 13th, 2020]
- What Is Sermorelin Therapy? Frequently Asked Questions [Last Updated On: February 12th, 2025] [Originally Added On: October 15th, 2020]
- Injectable Sermorelin with GHRP-6 for Men [Last Updated On: September 8th, 2024] [Originally Added On: November 2nd, 2020]
- FAQ Sermorelin and Wikipedia About Sermorelin: What are Sermorelin Acetate Injections? [Last Updated On: February 17th, 2025] [Originally Added On: November 3rd, 2020]
- Sermorelin Acetate Information [Last Updated On: October 12th, 2024] [Originally Added On: November 6th, 2020]
- Sermorelin Side Effects Explained [Last Updated On: February 10th, 2025] [Originally Added On: November 17th, 2020]
- HGH Side Effects: Risks and Dangers of Growth Hormone Therapy [Last Updated On: February 11th, 2025] [Originally Added On: November 19th, 2020]
- Ipamorelin Therapy for Human Growth Hormone Deficiency [Last Updated On: September 4th, 2024] [Originally Added On: November 24th, 2020]
- Improve Body Composition with Sermorelin Acetate [Last Updated On: October 26th, 2024] [Originally Added On: December 26th, 2020]
- How Can Sermorelin Help With Weight Loss ? [Last Updated On: April 8th, 2025] [Originally Added On: January 13th, 2021]
- What are the Benefits of Injectable Sermorelin Acetate? [Last Updated On: October 25th, 2024] [Originally Added On: January 20th, 2021]
- Buy Sermorelin Acetate Injections From a USA Clinic: HGH, testosterone, Sermorelin, HRT (Hormone replacement therapy) [Last Updated On: December 20th, 2024] [Originally Added On: February 8th, 2021]
- Sermorelin Therapy 21st Century Injectable Human Growth Hormone Restoration [Last Updated On: October 7th, 2024] [Originally Added On: February 16th, 2021]
- How Much Does Sermorelin Acetate Cost and How Does It Compare to HGH Therapy? [Last Updated On: April 11th, 2025] [Originally Added On: May 3rd, 2021]
- Buy Sermorelin -- What to do When Sermorelin Arrives in the Postal Mail [Last Updated On: February 16th, 2025] [Originally Added On: May 9th, 2021]
- The Importance of IGF-1 — Boosting IGF-1 Levels with Sermorelin and Prescription HGH [Last Updated On: December 26th, 2024] [Originally Added On: May 14th, 2021]
- Age Management with Sermorelin Injection Treatment [Last Updated On: March 7th, 2025] [Originally Added On: May 23rd, 2021]
- Sermorelin Acetate FAQ and Wiki Guide [Last Updated On: October 10th, 2024] [Originally Added On: May 25th, 2021]
- Buy Sermorelin: How Can I Legitimately Get a Sermorelin Prescription? [Last Updated On: November 23rd, 2024] [Originally Added On: May 30th, 2021]
- The Most Common Symptoms Associated with Sermorelin Acetate Injections [Last Updated On: September 27th, 2024] [Originally Added On: September 19th, 2021]
- Anti-Aging Benefits from Hormone Balance [Last Updated On: January 18th, 2025] [Originally Added On: March 22nd, 2022]
- How Can I Decide Between Sermorelin and HGH Injections? [Last Updated On: December 31st, 2024] [Originally Added On: January 25th, 2023]
- The Missing Vials Mystery [Last Updated On: March 31st, 2025] [Originally Added On: August 23rd, 2023]
- Gain Muscle and Improve Bodybuilding Results with Sermorelin Acetate Injections [Last Updated On: November 24th, 2024] [Originally Added On: March 9th, 2024]