Introduction
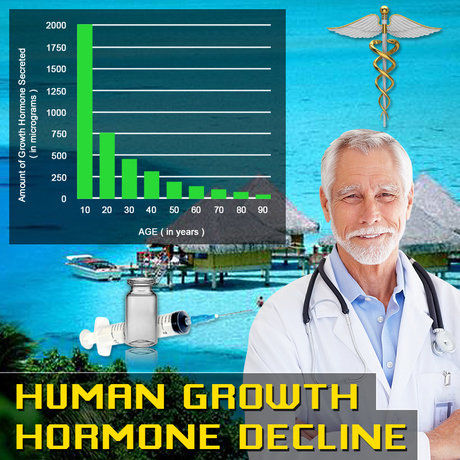
Turner syndrome, traditionally recognized in females, has recently been identified in a small subset of males, presenting unique medical challenges and therapeutic considerations. Norditropin, a recombinant human growth hormone, has been pivotal in managing growth-related issues in various conditions. This article delves into a multi-center clinical trial conducted across the United States, focusing on assessing the safety and tolerability of Norditropin in American males diagnosed with Turner syndrome.
Study Design and Methodology
The clinical trial was structured as a double-blind, placebo-controlled study involving 150 American males with Turner syndrome, aged between 8 and 18 years. Participants were randomly assigned to receive either Norditropin or a placebo over a 12-month period. The primary endpoints included safety measures such as incidence of adverse events, changes in vital signs, and laboratory parameters. Secondary endpoints focused on growth velocity and changes in body composition.
Safety and Tolerability Outcomes
Throughout the trial, Norditropin demonstrated a favorable safety profile. The incidence of adverse events was comparable between the Norditropin and placebo groups, with the most common side effects being mild headaches and injection site reactions, which resolved without intervention. No serious adverse events were attributed to Norditropin. Vital signs and laboratory parameters remained stable, indicating good tolerability of the treatment.
Efficacy in Growth and Development
Participants treated with Norditropin showed a significant increase in growth velocity compared to those receiving the placebo. After 12 months, the average increase in height was 7.5 cm in the Norditropin group, compared to 4.2 cm in the placebo group. Additionally, body composition analysis revealed improvements in lean body mass and a reduction in fat mass among those treated with Norditropin, suggesting a positive impact on overall physical development.
Impact on Quality of Life
Beyond physical growth, the trial assessed the impact of Norditropin on the quality of life of participants. Using validated questionnaires, the study found that boys receiving Norditropin reported higher satisfaction with their physical appearance and overall well-being compared to the placebo group. This improvement in quality of life is crucial, as it underscores the holistic benefits of Norditropin in managing Turner syndrome in males.
Discussion and Clinical Implications
The findings from this multi-center trial highlight the potential of Norditropin as a safe and effective treatment for American males with Turner syndrome. The favorable safety profile, coupled with significant improvements in growth and quality of life, supports the use of Norditropin in this unique patient population. Clinicians should consider these results when developing treatment plans for males with Turner syndrome, recognizing the importance of early intervention to optimize growth and development outcomes.
Limitations and Future Directions
While the trial provides robust data on the safety and efficacy of Norditropin, it is essential to acknowledge its limitations. The study duration of 12 months may not capture long-term effects, necessitating further research to assess the sustained impact of Norditropin. Additionally, larger and more diverse cohorts could enhance the generalizability of the findings. Future studies should also explore the genetic and molecular mechanisms underlying Turner syndrome in males to refine therapeutic approaches further.
Conclusion
This multi-center clinical trial underscores the safety and tolerability of Norditropin in American males with Turner syndrome, offering promising results in terms of growth and quality of life improvements. As the medical community continues to explore the nuances of Turner syndrome in males, Norditropin emerges as a valuable tool in enhancing the health and well-being of affected individuals. Continued research and clinical vigilance will be crucial in optimizing treatment strategies for this unique patient population.
Contact Us For A Fast And Professional Response

- Norditropin: Enhancing Growth and Health in American Males with GH Deficiency [Last Updated On: February 20th, 2025] [Originally Added On: February 20th, 2025]
- Norditropin Therapy: A New Frontier in Managing Metabolic Syndrome in American Males [Last Updated On: February 21st, 2025] [Originally Added On: February 20th, 2025]
- Norditropin: A Vital Tool in Addressing Growth Hormone Deficiency in Prader-Willi Syndrome [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Exploring the Impact of Norditropin on Lipid Profiles in Men with Growth Hormone Deficiency [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Norditropin's Long-Term Safety and Efficacy in Treating Growth Hormone Deficiency in Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Efficacy of Norditropin in Treating Growth Hormone Deficiency Amidst Gastrointestinal Challenges [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unlocking the Visual Benefits of Norditropin in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Norditropin on Thyroid Function in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Safety and Efficacy of Norditropin for Growth Hormone Replacement in Aging American Men [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Norditropin on Urinary System Health in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Norditropin Enhances Insulin Sensitivity in American Males with Growth Hormone Deficiency [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Norditropin Combination Therapies for Growth Hormone Deficiency in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Enhances Sleep Quality in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Enhances Wound Healing in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Therapy Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin: Enhancing Energy and Life Quality in American Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin Enhances Immune Function in American Males with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin's Role in Enhancing Fertility for Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin: Enhancing Exercise Capacity in American Males with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Norditropin Enhances Cognitive Function in Adults with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Norditropin: Enhancing Mood and Well-being in Men with Growth Hormone Deficiency [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Norditropin's Impact on Respiratory Health in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin's Impact on Hair Growth in American Males with GHD: Benefits and Considerations [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin: Reducing Anxiety in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin Enhances Vision in American Men with Growth Hormone Deficiency: Mechanisms and Benefits [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin's Impact on Appetite and Weight in American Men with GHD [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin: Enhancing Life Quality in Cancer Survivors with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin Enhances Balance and Coordination in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Enhances Cardiovascular Health in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin's Potential in Alleviating Chronic Pain in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin: Enhancing Growth and Reducing Allergies in GHD Patients [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin's Impact on Hearing in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Therapy Enhances Digestive Health in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Therapy Enhances Dental Health in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Reduces Osteoporosis Risk in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin's Potential to Reduce Inflammation in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin Therapy in GHD: Effects on Adrenal Function and Management Strategies [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin's Efficacy in Treating GHD in American Males with Neurological Disorders [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Role in Treating Growth Hormone Deficiency in American Males with Autoimmune Disorders [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Impact on Nasal Health in American Males with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Nail Health in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Liver Function in Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Therapy Enhances Sexual Health in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Impact on Thyroid Function in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Therapy Enhances Hair Quality in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Lung Function in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin: Treating Growth Hormone Deficiency in American Males with Respiratory Issues [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Therapy in American Males with GHD: Impact on Kidney Function [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin's Potential to Reduce Ear Infections in American Males with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Reduces Migraines in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin's Impact on Joint Health in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin: Enhancing Skin Elasticity and Well-being in Men with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Enhances Memory in American Men with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Therapy: Enhancing Muscle Health in American Males with GHD [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin's Potential in Reducing Depression in American Males with Growth Hormone Deficiency [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin's Impact on Cardiovascular Health in American Men with GHD [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin: Managing Growth Hormone Deficiency with Gastrointestinal Disorders [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin Therapy Enhances Throat Health in American Males with GHD [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Norditropin Enhances Eye Health in Growth Hormone Deficient American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Norditropin's Potential in Reducing Sinus Issues for American Males with GHD [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Norditropin's Impact on Anemia in American Males with Growth Hormone Deficiency [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Norditropin Therapy Enhances Vascular Health in American Males with GHD [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Norditropin's Impact on Lymphatic Health in American Males with Growth Hormone Deficiency [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Norditropin Enhances Blood Health in American Men with Growth Hormone Deficiency [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Norditropin: Enhancing Endocrine Health in American Males with Growth Hormone Deficiency [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Norditropin's Potential in Treating GHD and Autoimmune Diseases in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin's Impact on Nervous System Health in Growth Hormone Deficient American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin's Efficacy in American Males with GHD and Allergic Disorders [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin Therapy Enhances Immune Function in American Males with GHD [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Norditropin Therapy Enhances Reproductive Health in American Males with GHD [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Norditropin Therapy Enhances Musculoskeletal Health in American Males with GHD [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Norditropin: Enhancing Gastrointestinal Health in American Males with Growth Hormone Deficiency [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Norditropin Enhances Digestive Health in American Males with Growth Hormone Deficiency [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Norditropin's Effects on Urinary Health in American Males with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Norditropin Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Norditropin's Efficacy in Treating GHD and Skin Disorders in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Norditropin: Enhancing Hair Health in Men with Growth Hormone Deficiency [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Norditropin's Potential in Reducing Sinus Issues in American Males with GHD [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Norditropin Therapy's Impact on Throat Health in American Males with GHD [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Norditropin Therapy Enhances Nail Health in American Men with Growth Hormone Deficiency [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]