Introduction
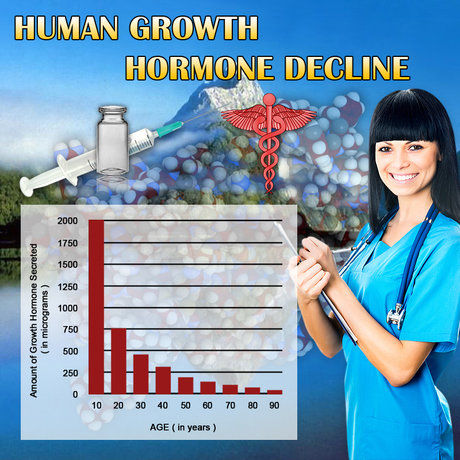
Kyzatrex, a novel oral testosterone replacement therapy, has emerged as a promising treatment option for hypogonadism in men. Understanding its pharmacokinetic properties is crucial for optimizing therapeutic outcomes and ensuring patient safety. This article delves into a comprehensive study exploring the pharmacokinetics of Kyzatrex oral capsules in American men, examining both single-dose and multiple-dose regimens. The findings provide valuable insights into the drug's absorption, distribution, metabolism, and excretion, offering a foundation for its clinical application.
Study Design and Methodology
The study was designed to evaluate the pharmacokinetics of Kyzatrex in healthy American male volunteers. Participants were divided into two groups: one receiving a single dose and the other a multiple-dose regimen over a specified period. Blood samples were collected at predetermined intervals to assess plasma testosterone concentrations. The study adhered to rigorous ethical standards, with informed consent obtained from all participants.
Single-Dose Pharmacokinetics
In the single-dose arm, participants received a standardized dose of Kyzatrex. The pharmacokinetic analysis revealed a rapid absorption phase, with peak plasma concentrations achieved within 2 to 4 hours post-administration. The mean maximum concentration (Cmax) and area under the curve (AUC) values indicated efficient absorption and systemic exposure. The elimination half-life was approximately 10 hours, suggesting a sustained release profile suitable for once-daily dosing.
Multiple-Dose Pharmacokinetics
The multiple-dose regimen provided insights into the steady-state pharmacokinetics of Kyzatrex. Participants received daily doses for two weeks, with blood sampling conducted at regular intervals. The data demonstrated a consistent increase in plasma testosterone levels, reaching a steady state by day 7. The steady-state Cmax and AUC values were higher than those observed with single dosing, reflecting cumulative drug exposure. Notably, the pharmacokinetic profile remained stable over the dosing period, indicating predictable drug behavior.
Comparison of Single and Multiple Dosing
Comparing the pharmacokinetic profiles from single and multiple dosing regimens highlighted significant differences. While single dosing provided an initial spike in testosterone levels, multiple dosing resulted in a more sustained and stable concentration. This finding underscores the importance of continuous dosing to maintain therapeutic levels, particularly in the context of testosterone replacement therapy.
Safety and Tolerability
Throughout the study, Kyzatrex was well-tolerated by participants, with no serious adverse events reported. Common side effects included mild headache and gastrointestinal discomfort, which resolved without intervention. The safety profile supports the use of Kyzatrex as a viable option for testosterone replacement in American men.
Clinical Implications
The pharmacokinetic data from this study have significant implications for the clinical use of Kyzatrex. The rapid absorption and sustained release characteristics suggest that once-daily dosing can effectively maintain therapeutic testosterone levels. Clinicians should consider these findings when prescribing Kyzatrex, tailoring the regimen to individual patient needs and monitoring for potential side effects.
Future Directions
Further research is warranted to explore the long-term effects of Kyzatrex and its efficacy in diverse patient populations. Additional studies could investigate the impact of food intake on absorption and the potential for drug interactions. Such research will enhance our understanding of Kyzatrex's role in testosterone replacement therapy and guide its optimal use in clinical practice.
Conclusion
This study provides a comprehensive analysis of the pharmacokinetics of Kyzatrex oral capsules in American men, offering valuable insights into its single-dose and multiple-dose profiles. The findings underscore the drug's potential as an effective and well-tolerated option for testosterone replacement therapy. As we continue to explore Kyzatrex's clinical applications, these pharmacokinetic data will serve as a crucial reference for healthcare professionals and researchers alike.
Contact Us For A Fast And Professional Response

- Optimizing Kyzatrex Efficacy: Diet, Lifestyle Tips for American Males with Hypogonadism [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Kyzatrex: Revolutionizing Testosterone Therapy for American Men's Health [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Kyzatrex: Oral Testosterone Therapy Enhancing Athletic Performance and Recovery in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Kyzatrex: Revolutionizing Testosterone Therapy for American Men with Oral Capsules [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Kyzatrex: A New Oral Testosterone Therapy and Its Side Effects Management [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Kyzatrex: Oral Testosterone Therapy Enhancing Life Quality in Aging American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Kyzatrex: Enhancing Fertility and Reproductive Health in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Impact on Prostate Health in Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Kyzatrex: Oral Testosterone Therapy Enhances Psychological Well-being in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Kyzatrex: Oral Testosterone Therapy's Impact on American Men's Health [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Kyzatrex: Oral Testosterone Therapy's Economic Impact on Men's Healthcare [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Kyzatrex: Oral Testosterone Therapy Boosts Energy in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Kyzatrex: Enhancing Cognitive Function in American Men with Oral Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Kyzatrex Oral Capsules: A Convenient Alternative to Injectable Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Kyzatrex: Enhancing Sleep Quality in American Men with Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Kyzatrex: Oral Testosterone Therapy for Hypogonadism in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Kyzatrex: Enhancing Emotional Health in American Males Through Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Kyzatrex: Enhancing Skin Health and Vitality in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Diabetes Management in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Kyzatrex: Revolutionizing Testosterone Therapy for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Kyzatrex: Revolutionizing Men's Health with Oral Testosterone Therapy in the U.S. [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex: Oral Testosterone Therapy for Chronic Conditions in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex: Oral Testosterone Therapy for American Males - Efficacy, Safety, and Challenges [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Cardiovascular Implications for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex: Oral Testosterone Therapy Revolutionizes Men's Preventive Health Care [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex Therapy: Lifestyle Adjustments for Optimal Testosterone Replacement in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Kyzatrex: Oral Testosterone Therapy for Hypogonadism in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Genetic Variations Impact Kyzatrex Efficacy in American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Enhancing American Men's Health Through Monitoring and Tailored Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Advancing Men's Health with Essential Regular Check-Ups [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Oral Testosterone Therapy Enhances Immune Function in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Oral Testosterone Therapy's Impact on Male Hair Growth and Loss [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Oral Testosterone Therapy for American Males - Enhancing Health Through Communication [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Oral Testosterone Therapy Revolutionizing Men's Health in the US [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex Capsules: Enhancing Joint Health and Mobility in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Enhancing Muscle Mass and Reducing Fat in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Impact on Eye Health in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Impact on Liver Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Strategies to Minimize Side Effects in Hypogonadism Treatment [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Oral Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Enhancing Weight Management in Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Holistic Treatment for Testosterone Deficiency in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: A Convenient, Discreet Oral Solution for Testosterone Deficiency in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Kyzatrex: Oral Testosterone Therapy and the Importance of Regular Blood Monitoring [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Kyzatrex: Enhancing Recovery in American Men with Oral Testosterone Capsules [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Kyzatrex: Oral Testosterone Therapy's Impact on Men's Mental Health in the U.S. [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Impact on Kidney Health in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Kyzatrex: Managing Blood Pressure in Testosterone Therapy for Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Kyzatrex: Optimizing Hypogonadism Treatment with Realistic Expectations and Holistic Care [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Kyzatrex: Enhancing Physical Endurance in American Males via Oral Testosterone Therapy [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Kyzatrex: A Novel Oral Capsule Enhancing Digestive Health in American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Kyzatrex: Oral Testosterone Therapy Enhances Post-Surgical Recovery in American Males [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Kyzatrex Oral Therapy: Enhancing Efficacy and Safety Through Hydration [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Respiratory Health Implications for American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Kyzatrex: Impacts on Dental Health and Preventive Measures for American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Kyzatrex: Advancing Mental Health Treatment for American Men with Oral Capsules [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Kyzatrex and Hearing Health: Risks and Monitoring for American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Kyzatrex Therapy: Managing Stress for Optimal Testosterone Treatment in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Kyzatrex: A New Oral Therapy for Autoimmune Diseases in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Kyzatrex: Enhancing Metabolic Health in American Males with Oral Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Kyzatrex: A Multifaceted Approach to Allergy Management for American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Kyzatrex Oral Capsules: Enhancing Immune Health in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Kyzatrex: A Novel Oral Therapy Enhancing Sleep Quality in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Kyzatrex: Oral Testosterone Therapy and Its Impact on Vision Health in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Kyzatrex: Managing Skin Sensitivity in Oral Testosterone Therapy for American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Kyzatrex Oral Capsules: Enhancing Exercise Recovery for American Men [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Kyzatrex: Revolutionizing Testosterone Deficiency Treatment in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Kyzatrex: Oral Testosterone Therapy's Gastrointestinal Impact and Management in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Kyzatrex: Oral Testosterone Therapy for American Males with Hypogonadism [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Kyzatrex Therapy: Enhancing Efficacy with Balanced Diet and Nutrition for American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Kyzatrex: A Novel Oral Solution for Chronic Pain Management in American Males [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Kyzatrex: Enhancing Neurological Health in American Males with Oral Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Kyzatrex: Oral Testosterone Therapy's Impact on American Males' Endocrine System [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Kyzatrex: Oral Testosterone Therapy for Hypogonadism - Importance of Regular Monitoring [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Kyzatrex: A Targeted Oral Solution for Inflammation in American Men [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Kyzatrex: Oral Testosterone Therapy's Impact on Urinary Health in American Males [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Managing Kyzatrex Side Effects: Strategies for Hypogonadism Treatment in Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Kyzatrex: A Convenient Oral Solution for American Men with Low Testosterone [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Kyzatrex: A Revolutionary Oral TRT for Hypogonadism in American Males [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Kyzatrex Oral Capsules: A New Era in Testosterone Replacement Therapy for American Men [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]